In this tutorial, we build an advanced multi-agent pipeline that interprets integrated omics data, including transcriptomics, proteomics, and metabolomics, to uncover key biological insights. We begin by generating coherent synthetic datasets that mimic realistic biological trends and then move step by step through agents designed for statistical analysis, network inference, pathway enrichment, and drug repurposing. Each component contributes to a cumulative interpretation process that allows us to identify significant genes, infer causal links, and generate biologically sound hypotheses supported by data patterns. Check out the FULL CODES here.
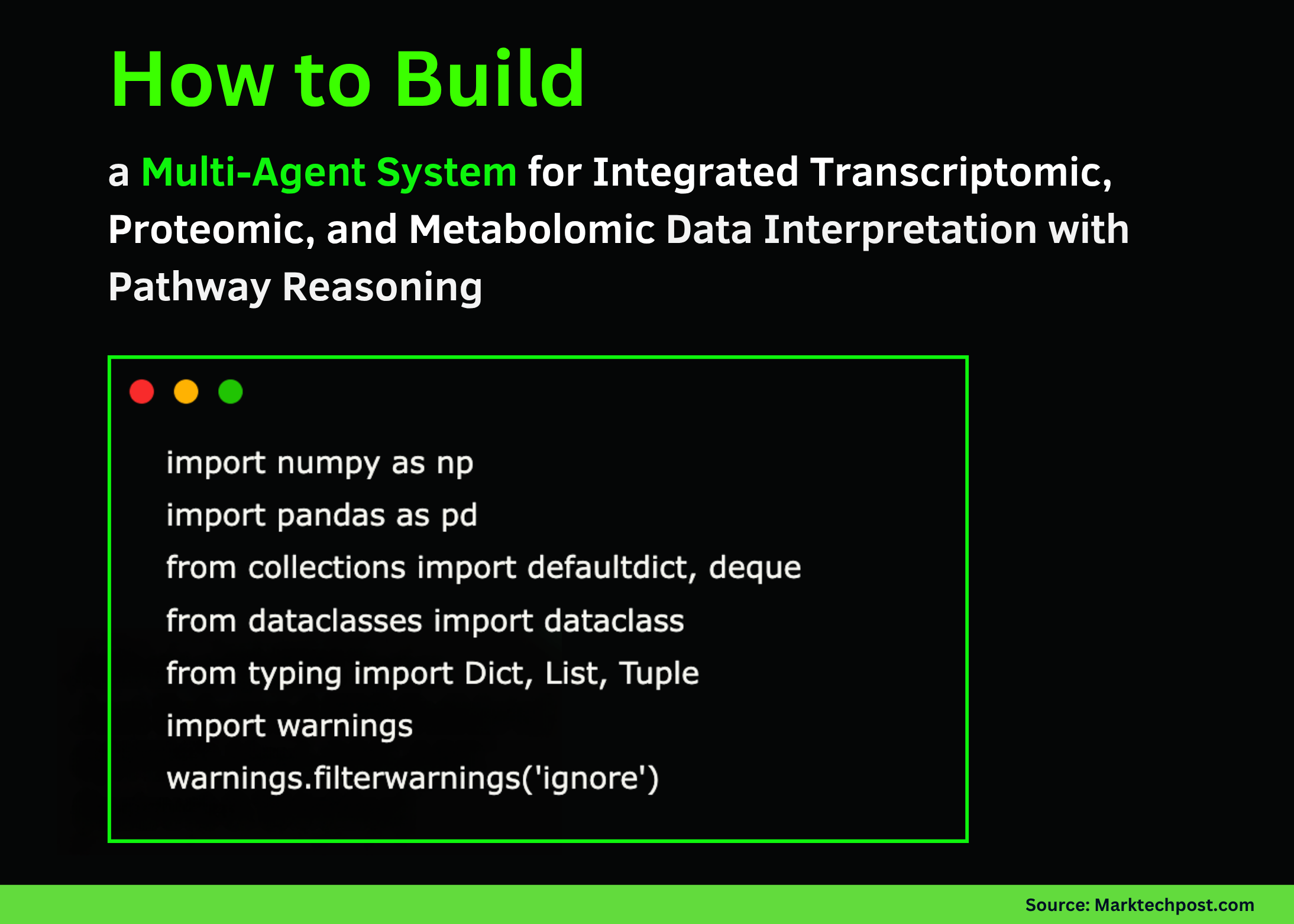
import numpy as np
import pandas as pd
from collections import defaultdict, deque
from dataclasses import dataclass
from typing import Dict, List, Tuple
import warnings
warnings.filterwarnings('ignore')
PATHWAY_DB = {
'Glycolysis': {'genes': ['HK2', 'PFKM', 'PKM', 'LDHA', 'GAPDH', 'ENO1'],
'metabolites': ['Glucose', 'G6P', 'F16BP', 'Pyruvate', 'Lactate'], 'score': 0},
'TCA_Cycle': {'genes': ['CS', 'IDH1', 'IDH2', 'OGDH', 'SDHA', 'MDH2'],
'metabolites': ['Citrate', 'Isocitrate', 'α-KG', 'Succinate', 'Malate'], 'score': 0},
'Oxidative_Phosphorylation': {'genes': ['NDUFA1', 'NDUFB5', 'COX5A', 'COX7A1', 'ATP5A1', 'ATP5B'],
'metabolites': ['ATP', 'ADP', 'NAD+', 'NADH'], 'score': 0},
'Fatty_Acid_Synthesis': {'genes': ['ACACA', 'FASN', 'SCD1', 'ACLY'],
'metabolites': ['Malonyl-CoA', 'Palmitate', 'Oleate'], 'score': 0},
'Fatty_Acid_Oxidation': {'genes': ['CPT1A', 'ACOX1', 'HADHA', 'ACADM'],
'metabolites': ['Acyl-CoA', 'Acetyl-CoA'], 'score': 0},
'Amino_Acid_Metabolism': {'genes': ['GOT1', 'GOT2', 'GLUD1', 'BCAT1', 'BCAT2'],
'metabolites': ['Glutamate', 'Glutamine', 'Alanine', 'Aspartate'], 'score': 0},
'Pentose_Phosphate': {'genes': ['G6PD', 'PGD', 'TKTL1'],
'metabolites': ['R5P', 'NADPH'], 'score': 0},
'Cell_Cycle_G1S': {'genes': ['CCND1', 'CDK4', 'CDK6', 'RB1', 'E2F1'], 'metabolites': [], 'score': 0},
'Cell_Cycle_G2M': {'genes': ['CCNB1', 'CDK1', 'CDC25C', 'WEE1'], 'metabolites': [], 'score': 0},
'Apoptosis': {'genes': ['BCL2', 'BAX', 'BID', 'CASP3', 'CASP8', 'CASP9'], 'metabolites': [], 'score': 0},
'mTOR_Signaling': {'genes': ['MTOR', 'RPTOR', 'RICTOR', 'AKT1', 'TSC1', 'TSC2'],
'metabolites': ['Leucine', 'ATP'], 'score': 0},
'HIF1_Signaling': {'genes': ['HIF1A', 'EPAS1', 'VEGFA', 'SLC2A1'], 'metabolites': ['Lactate'], 'score': 0},
'p53_Signaling': {'genes': ['TP53', 'MDM2', 'CDKN1A', 'BAX'], 'metabolites': [], 'score': 0},
'PI3K_AKT': {'genes': ['PIK3CA', 'AKT1', 'AKT2', 'PTEN', 'PDK1'], 'metabolites': [], 'score': 0},
}
GENE_INTERACTIONS = {
'HK2': ['PFKM', 'HIF1A', 'MTOR'], 'PFKM': ['PKM', 'HK2'], 'PKM': ['LDHA', 'HIF1A'],
'MTOR': ['AKT1', 'HIF1A', 'TSC2'], 'HIF1A': ['VEGFA', 'SLC2A1', 'PKM', 'LDHA'],
'TP53': ['MDM2', 'CDKN1A', 'BAX', 'CASP3'], 'AKT1': ['MTOR', 'TSC2', 'MDM2'],
'CCND1': ['CDK4', 'RB1'], 'CDK4': ['RB1'], 'RB1': ['E2F1'],
}
DRUG_TARGETS = {
'Metformin': ['NDUFA1'], 'Rapamycin': ['MTOR'], '2-DG': ['HK2'],
'Bevacizumab': ['VEGFA'], 'Palbociclib': ['CDK4', 'CDK6'], 'Nutlin-3': ['MDM2']
}
@dataclass
class OmicsProfile:
transcriptomics: pd.DataFrame
proteomics: pd.DataFrame
metabolomics: pd.DataFrame
metadata: DictWe set up the biological foundations of our system. We define pathway databases, gene–gene interactions, and drug–target mappings that serve as the reference network for all downstream analyses. We also import essential libraries and create a data class to store the multi-omics datasets in an organized format. Check out the FULL CODES here.
class AdvancedOmicsGenerator:
@staticmethod
def generate_coherent_omics(n_samples=30, n_timepoints=4, noise=0.2):
genes = list(set(g for p in PATHWAY_DB.values() for g in p['genes']))
metabolites = list(set(m for p in PATHWAY_DB.values() for m in p['metabolites'] if m))
proteins = [f"P_{g}" for g in genes]
n_control = n_samples // 2
samples_per_tp = n_samples // n_timepoints
trans = np.random.randn(len(genes), n_samples) * noise + 10
metab = np.random.randn(len(metabolites), n_samples) * noise + 5
for tp in range(n_timepoints):
start_idx = n_control + tp * samples_per_tp
end_idx = start_idx + samples_per_tp
progression = (tp + 1) / n_timepoints
for i, gene in enumerate(genes):
if gene in PATHWAY_DB['Glycolysis']['genes']:
trans[i, start_idx:end_idx] += np.random.uniform(1.5, 3.5) * progression
elif gene in PATHWAY_DB['Oxidative_Phosphorylation']['genes']:
trans[i, start_idx:end_idx] -= np.random.uniform(1, 2.5) * progression
elif gene in PATHWAY_DB['Cell_Cycle_G1S']['genes'] + PATHWAY_DB['Cell_Cycle_G2M']['genes']:
trans[i, start_idx:end_idx] += np.random.uniform(1, 2) * progression
elif gene in PATHWAY_DB['HIF1_Signaling']['genes']:
trans[i, start_idx:end_idx] += np.random.uniform(2, 4) * progression
elif gene in PATHWAY_DB['p53_Signaling']['genes']:
trans[i, start_idx:end_idx] -= np.random.uniform(0.5, 1.5) * progression
for i, met in enumerate(metabolites):
if met in ['Lactate', 'Pyruvate', 'G6P']:
metab[i, start_idx:end_idx] += np.random.uniform(1.5, 3) * progression
elif met in ['ATP', 'Citrate', 'Malate']:
metab[i, start_idx:end_idx] -= np.random.uniform(1, 2) * progression
elif met in ['NADPH']:
metab[i, start_idx:end_idx] += np.random.uniform(1, 2) * progression
prot = trans * 0.8 + np.random.randn(*trans.shape) * (noise * 2)
conditions = ['Control'] * n_control + [f'Disease_T{i//samples_per_tp}' for i in range(n_samples - n_control)]
trans_df = pd.DataFrame(trans, index=genes, columns=[f"S{i}_{c}" for i, c in enumerate(conditions)])
prot_df = pd.DataFrame(prot, index=proteins, columns=trans_df.columns)
metab_df = pd.DataFrame(metab, index=metabolites, columns=trans_df.columns)
metadata = {'conditions': conditions, 'n_timepoints': n_timepoints}
return OmicsProfile(trans_df, prot_df, metab_df, metadata)
class StatisticalAgent:
@staticmethod
def differential_analysis(data_df, control_samples, disease_samples):
control = data_df[control_samples]
disease = data_df[disease_samples]
fc = (disease.mean(axis=1) - control.mean(axis=1))
pooled_std = np.sqrt((control.var(axis=1) + disease.var(axis=1)) / 2)
t_stat = fc / (pooled_std + 1e-6)
p_values = 2 * (1 - np.minimum(np.abs(t_stat) / (np.abs(t_stat).max() + 1e-6), 0.999))
sorted_pvals = np.sort(p_values)
ranks = np.searchsorted(sorted_pvals, p_values) + 1
fdr = p_values * len(p_values) / ranks
return pd.DataFrame({'log2FC': fc, 't_stat': t_stat, 'p_value': p_values,
'FDR': np.minimum(fdr, 1.0), 'significant': (np.abs(fc) > 1.0) & (fdr < 0.05)}).sort_values('log2FC', ascending=False)
@staticmethod
def temporal_analysis(data_df, metadata):
timepoints = metadata['n_timepoints']
samples_per_tp = data_df.shape[1] // (timepoints + 1)
trends = {}
for gene in data_df.index:
means = []
for tp in range(timepoints):
start = samples_per_tp + tp * samples_per_tp
end = start + samples_per_tp
means.append(data_df.iloc[:, start:end].loc[gene].mean())
if len(means) > 1:
x = np.arange(len(means))
coeffs = np.polyfit(x, means, deg=min(2, len(means)-1))
trends[gene] = {'slope': coeffs[0] if len(coeffs) > 1 else 0, 'trajectory': means}
return trendsWe focus on generating synthetic but biologically coherent multi-omics data and performing the initial statistical analysis. We simulate disease progression across timepoints and compute fold changes, p-values, and FDR-corrected significance levels for genes, proteins, and metabolites. We also examine temporal trends to capture how expression values evolve over time. Check out the FULL CODES here.
class NetworkAnalysisAgent:
def __init__(self, interactions):
self.graph = interactions
def find_master_regulators(self, diff_genes):
sig_genes = diff_genes[diff_genes['significant']].index.tolist()
impact_scores = {}
for gene in sig_genes:
if gene in self.graph:
downstream = self._bfs_downstream(gene, max_depth=2)
sig_downstream = [g for g in downstream if g in sig_genes]
impact_scores[gene] = {
'downstream_count': len(downstream),
'sig_downstream': len(sig_downstream),
'score': len(sig_downstream) / (len(downstream) + 1),
'fc': diff_genes.loc[gene, 'log2FC']
}
return sorted(impact_scores.items(), key=lambda x: x[1]['score'], reverse=True)
def _bfs_downstream(self, start, max_depth=2):
visited, queue = set(), deque([(start, 0)])
downstream = []
while queue:
node, depth = queue.popleft()
if depth >= max_depth or node in visited:
continue
visited.add(node)
if node in self.graph:
for neighbor in self.graph[node]:
if neighbor not in visited:
downstream.append(neighbor)
queue.append((neighbor, depth + 1))
return downstream
def causal_inference(self, diff_trans, diff_prot, diff_metab):
causal_links = []
for gene in diff_trans[diff_trans['significant']].index:
gene_fc = diff_trans.loc[gene, 'log2FC']
protein = f"P_{gene}"
if protein in diff_prot.index:
prot_fc = diff_prot.loc[protein, 'log2FC']
correlation = np.sign(gene_fc) == np.sign(prot_fc)
if correlation and abs(prot_fc) > 0.5:
causal_links.append(('transcription', gene, protein, gene_fc, prot_fc))
for pathway, content in PATHWAY_DB.items():
if gene in content['genes']:
for metab in content['metabolites']:
if metab in diff_metab.index and diff_metab.loc[metab, 'significant']:
metab_fc = diff_metab.loc[metab, 'log2FC']
causal_links.append(('enzymatic', gene, metab, gene_fc, metab_fc))
return causal_linksWe implement the network analysis agent that identifies master regulators and infers causal relationships. We utilize graph traversal to assess the impact of each gene on others and to identify connections between transcriptional, proteomic, and metabolic layers. This helps us understand which nodes have the greatest downstream impact on biological processes. Check out the FULL CODES here.
class PathwayEnrichmentAgent:
def __init__(self, pathway_db, interactions):
self.pathway_db = pathway_db
self.interactions = interactions
def topology_weighted_enrichment(self, diff_genes, diff_metab, network_agent):
enriched = {}
for pathway, content in self.pathway_db.items():
sig_genes = [g for g in content['genes'] if g in diff_genes.index and diff_genes.loc[g, 'significant']]
weighted_score = 0
for gene in sig_genes:
base_score = abs(diff_genes.loc[gene, 'log2FC'])
downstream = network_agent._bfs_downstream(gene, max_depth=1)
centrality = len(downstream) / 10
weighted_score += base_score * (1 + centrality)
sig_metabs = [m for m in content['metabolites'] if m in diff_metab.index and diff_metab.loc[m, 'significant']]
metab_score = sum(abs(diff_metab.loc[m, 'log2FC']) for m in sig_metabs)
total_score = (weighted_score + metab_score * 2) / max(len(content['genes']) + len(content['metabolites']), 1)
if total_score > 0.5:
enriched[pathway] = {'score': total_score, 'genes': sig_genes, 'metabolites': sig_metabs,
'gene_fc': {g: diff_genes.loc[g, 'log2FC'] for g in sig_genes},
'metab_fc': {m: diff_metab.loc[m, 'log2FC'] for m in sig_metabs},
'coherence': self._pathway_coherence(sig_genes, diff_genes)}
return enriched
def _pathway_coherence(self, genes, diff_genes):
if len(genes) < 2:
return 0
fcs = [diff_genes.loc[g, 'log2FC'] for g in genes]
same_direction = sum(1 for fc in fcs if np.sign(fc) == np.sign(fcs[0]))
return same_direction / len(genes)We add pathway-level reasoning by incorporating topology-weighted enrichment analysis. We assess which biological pathways exhibit significant activation or suppression and weight them according to network centrality to reflect their broader influence. The agent also evaluates pathway coherence, indicating whether genes in a pathway exhibit consistent directional movement. Check out the FULL CODES here.
class DrugRepurposingAgent:
def __init__(self, drug_db):
self.drug_db = drug_db
def predict_drug_response(self, diff_genes, master_regulators):
predictions = []
for drug, targets in self.drug_db.items():
score = 0
affected_targets = []
for target in targets:
if target in diff_genes.index:
fc = diff_genes.loc[target, 'log2FC']
is_sig = diff_genes.loc[target, 'significant']
if is_sig:
drug_benefit = -fc if fc > 0 else 0
score += drug_benefit
affected_targets.append((target, fc))
if target in [mr[0] for mr in master_regulators[:5]]:
score += 2
if score > 0:
predictions.append({
'drug': drug,
'score': score,
'targets': affected_targets,
'mechanism': 'Inhibition of upregulated pathway'
})
return sorted(predictions, key=lambda x: x['score'], reverse=True)
class AIHypothesisEngine:
def generate_comprehensive_report(self, omics_data, analysis_results):
report = ["="*80, "ADVANCED MULTI-OMICS INTERPRETATION REPORT", "="*80, ""]
trends = analysis_results['temporal']
top_trends = sorted(trends.items(), key=lambda x: abs(x[1]['slope']), reverse=True)[:5]
report.append(" TEMPORAL DYNAMICS ANALYSIS:")
for gene, data in top_trends:
direction = "↑ Increasing" if data['slope'] > 0 else "↓ Decreasing"
report.append(f" {gene}: {direction} (slope: {data['slope']:.3f})")
report.append("n
TEMPORAL DYNAMICS ANALYSIS:")
for gene, data in top_trends:
direction = "↑ Increasing" if data['slope'] > 0 else "↓ Decreasing"
report.append(f" {gene}: {direction} (slope: {data['slope']:.3f})")
report.append("n MASTER REGULATORS (Top 5):")
for gene, data in analysis_results['master_regs'][:5]:
report.append(f" • {gene}: Controls {data['sig_downstream']} dysregulated genes (FC: {data['fc']:+.2f}, Impact: {data['score']:.3f})")
report.append("n
MASTER REGULATORS (Top 5):")
for gene, data in analysis_results['master_regs'][:5]:
report.append(f" • {gene}: Controls {data['sig_downstream']} dysregulated genes (FC: {data['fc']:+.2f}, Impact: {data['score']:.3f})")
report.append("n ENRICHED PATHWAYS:")
for pathway, data in sorted(analysis_results['pathways'].items(), key=lambda x: x[1]['score'], reverse=True):
report.append(f"n ► {pathway} (Score: {data['score']:.3f}, Coherence: {data['coherence']:.2f})")
report.append(f" Genes: {', '.join(data['genes'][:6])}")
if data['metabolites']:
report.append(f" Metabolites: {', '.join(data['metabolites'][:4])}")
report.append("n
ENRICHED PATHWAYS:")
for pathway, data in sorted(analysis_results['pathways'].items(), key=lambda x: x[1]['score'], reverse=True):
report.append(f"n ► {pathway} (Score: {data['score']:.3f}, Coherence: {data['coherence']:.2f})")
report.append(f" Genes: {', '.join(data['genes'][:6])}")
if data['metabolites']:
report.append(f" Metabolites: {', '.join(data['metabolites'][:4])}")
report.append("n CAUSAL RELATIONSHIPS (Top 10):")
for link_type, source, target, fc1, fc2 in analysis_results['causal'][:10]:
report.append(f" {source} →[{link_type}]→ {target} (FC: {fc1:+.2f} → {fc2:+.2f})")
report.append("n
CAUSAL RELATIONSHIPS (Top 10):")
for link_type, source, target, fc1, fc2 in analysis_results['causal'][:10]:
report.append(f" {source} →[{link_type}]→ {target} (FC: {fc1:+.2f} → {fc2:+.2f})")
report.append("n DRUG REPURPOSING PREDICTIONS:")
for pred in analysis_results['drugs'][:5]:
report.append(f" • {pred['drug']} (Score: {pred['score']:.2f})")
report.append(f" Targets: {', '.join([f'{t[0]}({t[1]:+.1f})' for t in pred['targets']])}")
report.append("n
DRUG REPURPOSING PREDICTIONS:")
for pred in analysis_results['drugs'][:5]:
report.append(f" • {pred['drug']} (Score: {pred['score']:.2f})")
report.append(f" Targets: {', '.join([f'{t[0]}({t[1]:+.1f})' for t in pred['targets']])}")
report.append("n AI-GENERATED BIOLOGICAL HYPOTHESES:n")
for i, hyp in enumerate(self._generate_advanced_hypotheses(analysis_results), 1):
report.append(f"{i}. {hyp}n")
report.append("="*80)
return "n".join(report)
def _generate_advanced_hypotheses(self, results):
hypotheses = []
pathways = results['pathways']
if 'Glycolysis' in pathways and 'Oxidative_Phosphorylation' in pathways:
glyc = pathways['Glycolysis']['score']
oxphos = pathways['Oxidative_Phosphorylation']['score']
if glyc > oxphos * 1.5:
hypotheses.append(
"WARBURG EFFECT DETECTED: Aerobic glycolysis upregulation with oxidative phosphorylation suppression suggests metabolic reprogramming driven by HIF1A."
)
if 'Cell_Cycle_G1S' in pathways and 'mTOR_Signaling' in pathways:
hypotheses.append(
"PROLIFERATIVE SIGNATURE: Cell-cycle activation with mTOR signaling indicates anabolic reprogramming; dual CDK4/6 and mTOR inhibition may be effective."
)
if results['master_regs']:
top_mr = results['master_regs'][0]
hypotheses.append(
f"UPSTREAM REGULATOR: {top_mr[0]} controls {top_mr[1]['sig_downstream']} dysregulated genes; targeting this node can propagate network-wide correction."
)
trends = results['temporal']
progressive = [g for g, d in trends.items() if abs(d['slope']) > 0.5]
if len(progressive) > 5:
hypotheses.append(
f"PROGRESSIVE DYSREGULATION: {len(progressive)} genes show strong temporal shifts, indicating evolving pathology and benefit from early pathway intervention."
)
if 'HIF1_Signaling' in pathways:
hypotheses.append(
"HYPOXIA RESPONSE: HIF1 signaling suggests oxygen-poor microenvironment; anti-angiogenic strategies may normalize perfusion."
)
if 'p53_Signaling' in pathways:
hypotheses.append(
"TUMOR SUPPRESSOR LOSS: p53 pathway suppression suggests benefit from MDM2 inhibition if TP53 is wild-type."
)
return hypotheses if hypotheses else ["Complex multi-factorial dysregulation detected."]
AI-GENERATED BIOLOGICAL HYPOTHESES:n")
for i, hyp in enumerate(self._generate_advanced_hypotheses(analysis_results), 1):
report.append(f"{i}. {hyp}n")
report.append("="*80)
return "n".join(report)
def _generate_advanced_hypotheses(self, results):
hypotheses = []
pathways = results['pathways']
if 'Glycolysis' in pathways and 'Oxidative_Phosphorylation' in pathways:
glyc = pathways['Glycolysis']['score']
oxphos = pathways['Oxidative_Phosphorylation']['score']
if glyc > oxphos * 1.5:
hypotheses.append(
"WARBURG EFFECT DETECTED: Aerobic glycolysis upregulation with oxidative phosphorylation suppression suggests metabolic reprogramming driven by HIF1A."
)
if 'Cell_Cycle_G1S' in pathways and 'mTOR_Signaling' in pathways:
hypotheses.append(
"PROLIFERATIVE SIGNATURE: Cell-cycle activation with mTOR signaling indicates anabolic reprogramming; dual CDK4/6 and mTOR inhibition may be effective."
)
if results['master_regs']:
top_mr = results['master_regs'][0]
hypotheses.append(
f"UPSTREAM REGULATOR: {top_mr[0]} controls {top_mr[1]['sig_downstream']} dysregulated genes; targeting this node can propagate network-wide correction."
)
trends = results['temporal']
progressive = [g for g, d in trends.items() if abs(d['slope']) > 0.5]
if len(progressive) > 5:
hypotheses.append(
f"PROGRESSIVE DYSREGULATION: {len(progressive)} genes show strong temporal shifts, indicating evolving pathology and benefit from early pathway intervention."
)
if 'HIF1_Signaling' in pathways:
hypotheses.append(
"HYPOXIA RESPONSE: HIF1 signaling suggests oxygen-poor microenvironment; anti-angiogenic strategies may normalize perfusion."
)
if 'p53_Signaling' in pathways:
hypotheses.append(
"TUMOR SUPPRESSOR LOSS: p53 pathway suppression suggests benefit from MDM2 inhibition if TP53 is wild-type."
)
return hypotheses if hypotheses else ["Complex multi-factorial dysregulation detected."]We introduce drug repurposing and hypothesis generation agents. We score potential drugs based on the dysregulation of their targets and the network importance of affected genes, then compile interpretative hypotheses that link pathway activity to possible interventions. The report generation engine summarizes these findings in a structured, readable format. Check out the FULL CODES here.
def run_advanced_omics_interpretation():
print(" Initializing Advanced Multi-Agent Omics System...n")
omics = AdvancedOmicsGenerator.generate_coherent_omics()
print("
Initializing Advanced Multi-Agent Omics System...n")
omics = AdvancedOmicsGenerator.generate_coherent_omics()
print(" Generated multi-omics dataset")
stat_agent = StatisticalAgent()
control_samples = [c for c in omics.transcriptomics.columns if 'Control' in c]
disease_samples = [c for c in omics.transcriptomics.columns if 'Disease' in c]
diff_trans = stat_agent.differential_analysis(omics.transcriptomics, control_samples, disease_samples)
diff_prot = stat_agent.differential_analysis(omics.proteomics, control_samples, disease_samples)
diff_metab = stat_agent.differential_analysis(omics.metabolomics, control_samples, disease_samples)
temporal = stat_agent.temporal_analysis(omics.transcriptomics, omics.metadata)
network_agent = NetworkAnalysisAgent(GENE_INTERACTIONS)
master_regs = network_agent.find_master_regulators(diff_trans)
causal_links = network_agent.causal_inference(diff_trans, diff_prot, diff_metab)
pathway_agent = PathwayEnrichmentAgent(PATHWAY_DB, GENE_INTERACTIONS)
enriched = pathway_agent.topology_weighted_enrichment(diff_trans, diff_metab, network_agent)
drug_agent = DrugRepurposingAgent(DRUG_TARGETS)
drug_predictions = drug_agent.predict_drug_response(diff_trans, master_regs)
results = {
'temporal': temporal,
'master_regs': master_regs,
'causal': causal_links,
'pathways': enriched,
'drugs': drug_predictions
}
hypothesis_engine = AIHypothesisEngine()
report = hypothesis_engine.generate_comprehensive_report(omics, results)
print(report)
return omics, results
if __name__ == "__main__":
omics_data, analysis = run_advanced_omics_interpretation()
Generated multi-omics dataset")
stat_agent = StatisticalAgent()
control_samples = [c for c in omics.transcriptomics.columns if 'Control' in c]
disease_samples = [c for c in omics.transcriptomics.columns if 'Disease' in c]
diff_trans = stat_agent.differential_analysis(omics.transcriptomics, control_samples, disease_samples)
diff_prot = stat_agent.differential_analysis(omics.proteomics, control_samples, disease_samples)
diff_metab = stat_agent.differential_analysis(omics.metabolomics, control_samples, disease_samples)
temporal = stat_agent.temporal_analysis(omics.transcriptomics, omics.metadata)
network_agent = NetworkAnalysisAgent(GENE_INTERACTIONS)
master_regs = network_agent.find_master_regulators(diff_trans)
causal_links = network_agent.causal_inference(diff_trans, diff_prot, diff_metab)
pathway_agent = PathwayEnrichmentAgent(PATHWAY_DB, GENE_INTERACTIONS)
enriched = pathway_agent.topology_weighted_enrichment(diff_trans, diff_metab, network_agent)
drug_agent = DrugRepurposingAgent(DRUG_TARGETS)
drug_predictions = drug_agent.predict_drug_response(diff_trans, master_regs)
results = {
'temporal': temporal,
'master_regs': master_regs,
'causal': causal_links,
'pathways': enriched,
'drugs': drug_predictions
}
hypothesis_engine = AIHypothesisEngine()
report = hypothesis_engine.generate_comprehensive_report(omics, results)
print(report)
return omics, results
if __name__ == "__main__":
omics_data, analysis = run_advanced_omics_interpretation()We orchestrate the entire workflow, running all agents sequentially and aggregating their results into a comprehensive report. We execute the pipeline end-to-end, from data generation to insight generation, verifying that each component contributes to the overall interpretation. The final output provides an integrated multi-omics view with actionable insights.
In conclusion, this tutorial demonstrated how a structured, modular workflow can connect different layers of omics data into an interpretable analytical framework. By combining statistical reasoning, network topology, and biological context, we produced a comprehensive summary that highlights potential regulatory mechanisms and candidate therapeutic directions. The approach remains clear, data-driven, and adaptable for both simulated and real multi-omics datasets.
Check out the FULL CODES here. Feel free to check out our GitHub Page for Tutorials, Codes and Notebooks. Also, feel free to follow us on Twitter and don’t forget to join our 100k+ ML SubReddit and Subscribe to our Newsletter. Wait! are you on telegram? now you can join us on telegram as well.
The post Build a Multi-Agent System for Integrated Transcriptomic, Proteomic, and Metabolomic Data Interpretation with Pathway Reasoning appeared first on MarkTechPost.